An Overlooked but Effective Wound Care Methodology: Hydromechanical Therapy Revisited
Author: Lifei Guo, MD, PhD, FACS
View/Download PDF
Summary
Chronic wounds are frequently difficult, expensive to treat, and pose a significant burden on both the patient’s quality of life and health care system. Their recalcitrance to treatment stems from multiple factors, particularly the presence of bacterial biofilms within the wound bed. However, a commonly overlooked modality in the field of wound care, pressurized irrigation, offers an inexpensive mechanical debridement force capable of dislodging these biofilms that contribute to delayed healing of chronic wounds. We present here a single clinical case of a difficult nonhealing wound that had previously failed 3 months of negative-pressure wound therapy, a much more expensive modality. This chronic plantar foot wound was treated with daily application of hydromechanical therapy using tap water at home. It achieved a stable granulation surface, and with a small skin graft, healing with no recurrence seen at 15-month follow-up. We speculate that a combination of tissue stimulation and disruption of the wound surface biofilm contribute to improved healing, supporting a reevaluation for the use of pressurized irrigation in the treatment of chronic wounds. (Plast Reconstr Surg Glob Open 2018;6:e1883; doi: 10.1097/GOX.0000000000001883; Published online 3 August 2018.)
Introduction
The care of chronic wounds often presents a difficult challenge for health care practitioners due to their resistance to many of the current modalities and approaches to treatment. Presently, chronic wounds negatively affect patients’ quality of life in addition to incurring a huge financial burden on the U.S. health care system of over $25 billion per year.
Frequently, bacterial biofilms are a major offender in the delayed healing of chronic wounds through production of bacterial proteases and disruption of host immune cellular function.2–4 As a result, wounds persist in a state of chronic inflammation without progressing to stable healing. Pressurized irrigation offers a solution through its ability to mechanically debride and reduce bacterial colonization. It offers an inexpensive and easy to administer method of treating chronic wounds that can be accomplished in any wound treatment environment. Additionally, it offers further opportunities for cost savings through the use of tap water as an irrigation solution, as tap water has not been associated with increased risks for infection when compared with sterile saline.
Despite its efficacy and potential for cost savings, the field of wound care has frequently overlooked hydromechanical therapy for more expensive, often less effective modalities. In this article, we present a single clinical case treated with hydromechanical therapy using tap water. We intend to demonstrate that this traditional and inexpensive modality can promote significantly improved healing in nonhealing chronic wounds that have failed other more conventional and costly approaches.
Case Example
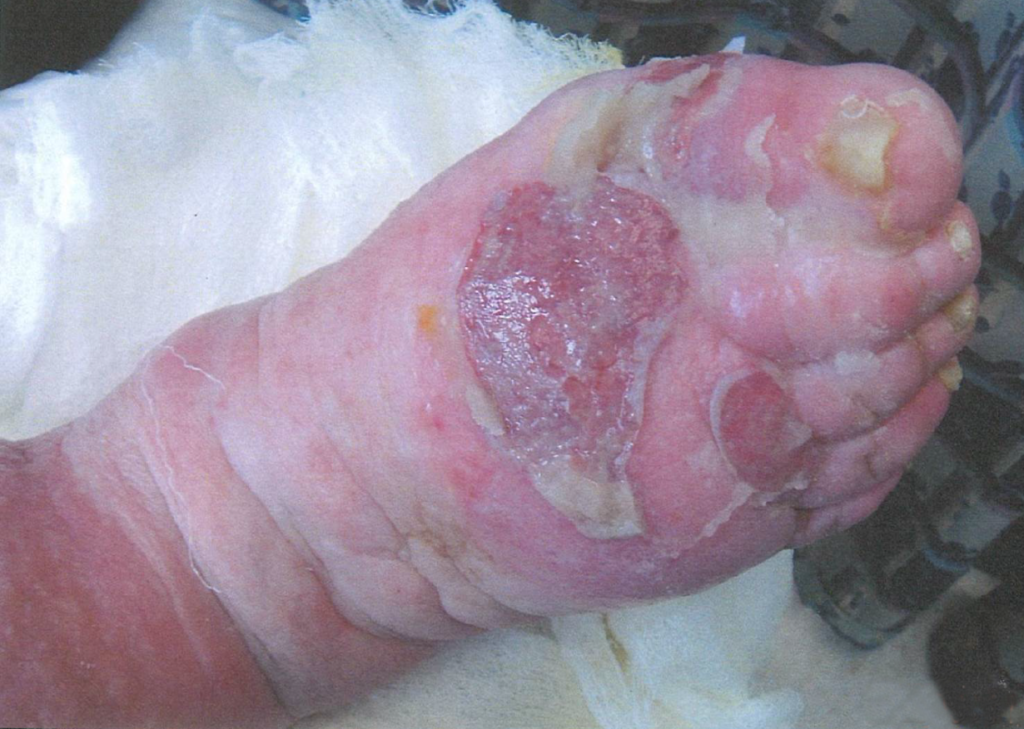
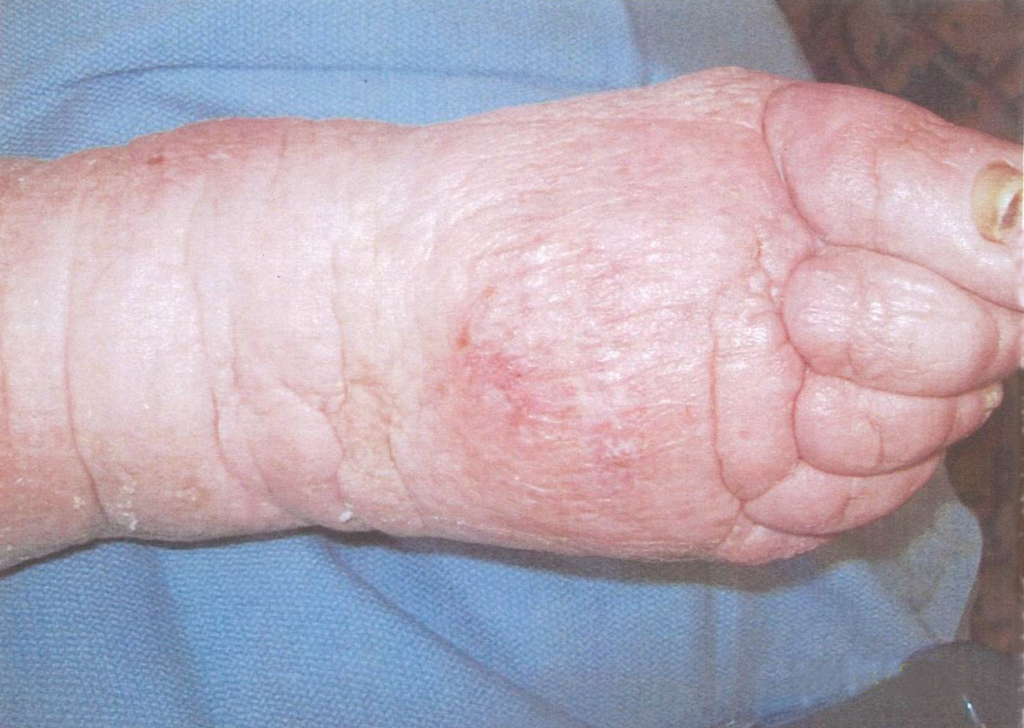
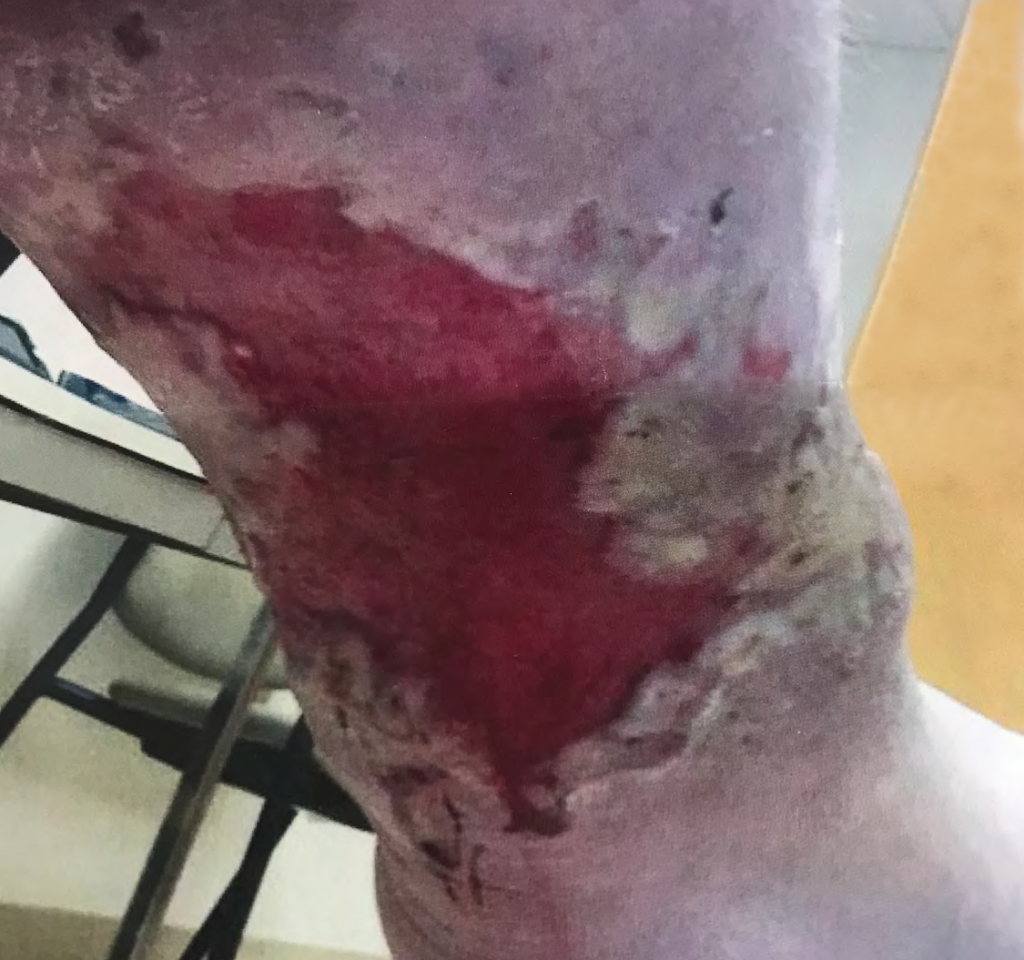
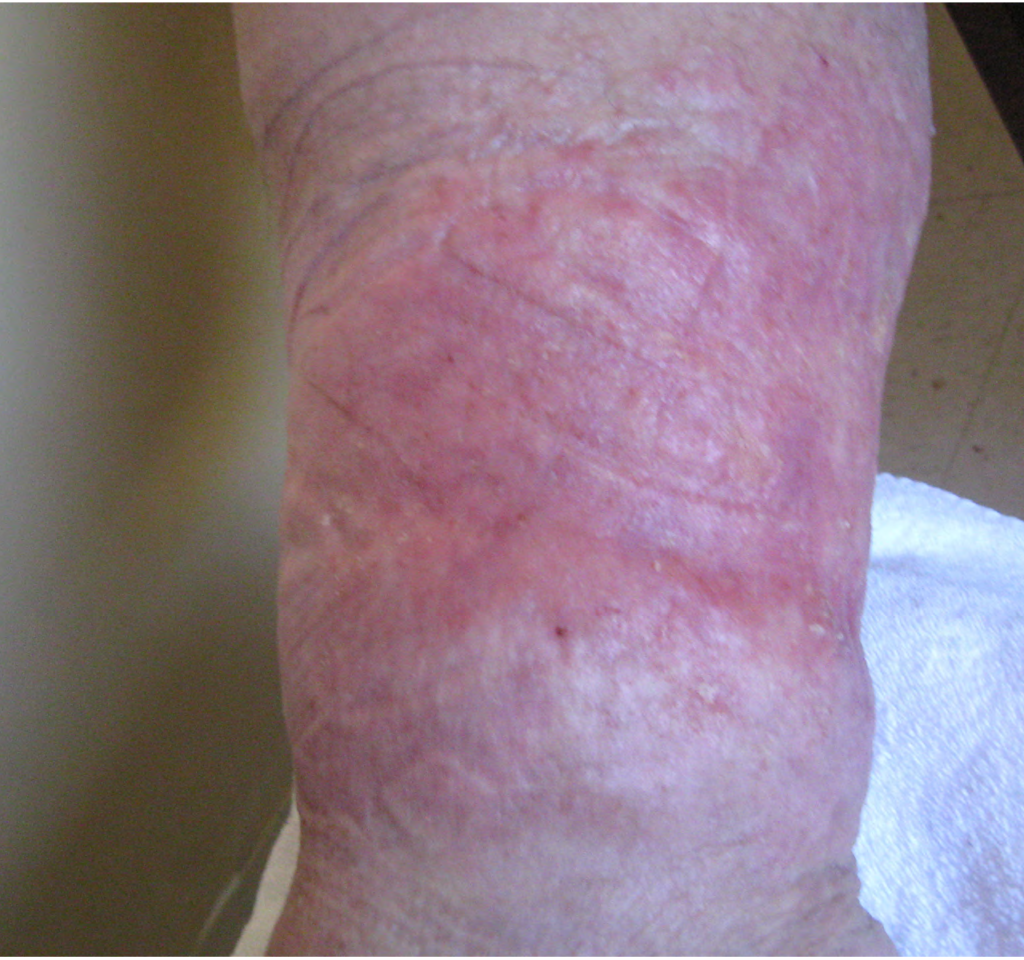
A 67-year-old ambulatory patient from the author’s practice with severe peripheral neuropathy and type I diabetes on insulin pump, with relatively good glycemic control, presented with a large chronic plantar foot wound (6.5×10cm) with significant plantar tendon and metatarsal head exposure that had failed 3 months of negative pressure therapy (Fig. 1). The patient self-administered twice daily 5-minute hydromechanical debridements using a home hand-held showerhead with tap water and wet-to-moist dressing changes. Degree of pressure administered was adjusted by the patient based on what flow of water was tolerable, minimizing pain and potential tissue damage while strong enough to dislodge devitalized tissue and adherent bacteria. No antibiotic was required. Within 2 months, the wound had significantly improved with healthy granulation tissue formation and was subsequently grafted with a full-thickness skin graft (2.5×7cm). At 15-month follow-up, the wound had achieved stable healing with no recurrence.
Discussion
We have presented here a representative case of a nonhealing chronic wound that achieved stable healing through the use of hydromechanical therapy, despite previous failed attempts with negative pressure wound therapy. Hydromechanical therapy has been utilized in operating rooms and emergency departments for years but offers an important asset to the field of chronic wound care through its ability to mechanically debride and cleanse wound surfaces.8–10 Additionally, this inexpensive and easily administrable modality exerts repetitive mechanical forces through continuous impact that is thought to promote tissue regeneration and achieve wound healing.
Currently, mechanical pressurized irrigation is primarily utilized in hospital settings for its ability to disrupt bacterial adherence and devitalized tissues, but its ease of application and minimal ancillary resources required to administer the therapy offer a convenient and affordable option for patients in the outpatient setting. Furthermore, while sterile saline irrigation is the most commonly used irrigation solution, other various cleansing solutions, such as tap water, have been suggested with studies reporting no significant difference in clinical infection rates in their treatments of acute wounds or chronic wounds. This offers an enormous opportunity to reduce costs associated with the care of chronic wounds.
Biofilms are a relatively new concept in the field of chronic wound treatment and consist of complex microbial communities embedded in a protective extracellular polymeric substance secreted by each biofilm bacteria. Current research suggests that while mechanical debridement is the best way to remove biofilm, any residual pathogen can reconstitute the biofilm within days. Unfortunately, while other popular chronic wound care modalities may promote tissue regeneration and decrease bacterial counts, including negative pressure wound therapy, they do not involve a frequent daily mechanical debridement force. Hydromechanical debridement, however, provides a potential mechanism for dislodging both devitalized tissue and its associated biofilm without incurring substantial damage to the surrounding healthy tissue when administered daily. The precise pressure utilized may vary from application to application, but the general consensus appears to suggest a pressure greater than 10 psi, which is just strong enough to remove unwanted materials on the wound surface, and less than 50 psi, above which there might exist concerns of tissue damage, patient intolerance, and bacterial injection into tissue. In addition, there is evidence that, similar to other mechanical forces such as suction and vibration, the percussive forces delivered by irrigation fluid upon a wound surface induce heightened granulation tissue formation and hasten wound closure.
Finally, in addition to its efficacy, hydromechanical therapy offers a tremendous opportunity to reduce costs associated with the treatment of chronic wounds. Due to the wide availability of water or saline and minimal training required to deliver this simple modality, hydromechanical debridement can be carried out in both home and facility settings. As the care of chronic wounds continues to contribute a large financial burden to the rapidly increasing costs of health care, this traditional approach of hydromechanical debridement should be revisited.
Looking for Chinks in the Armor of Bacterial Biofilms
Author: Don Monroe
View/Download PDF
David Davies stood at the hospital bedside of his greataunt, who had recently had all her toes amputated to try to prevent persistent wounds from spreading. It didn’t work; doctors would later amputate both of her feet, and she never returned to her independent life. As Davies thinks back to this episode from his high-school days, “I remember wondering ‘How come, in this era of antibiotics, was it not possible to treat what was obviously, to me, an infection?’”
Even today, such nonhealing wounds are common in people with late-stage diabetes like Davies’ great-aunt, who have poor circulation, as well as in people with compromised immune systems. Davies, now an associate professor at Binghamton University, says that many doctors treat these debilitating wounds as a problem with the patient, rather than a sign of infection. “There’s no excuse for it,” he says.
Wounds are just one example of the huge impact of bacterial biofilms. The United States National Institutes of Health says that 80% of chronic infections are biofilm related. Unlike the more familiar “planktonic” lifestyle, in which bacteria float or swim freely, in biofilms they surround themselves with a complex polymeric matrix, better known as “slime.” As it grows thicker, the film often includes many bacterial species and the matrix develops a complex structure. Traditional antibiotics are often ineffective. “We thought we had it all figured out,” Davies says, but the past 20 years have shown that researchers are still in the “dark ages” when it comes to understanding and controlling bacteria.
As biofilms, bacteria routinely foul industrial equipment and medical devices like catheters and implants, where they form dense layers that cling tightly to the artificial surfaces. They also occur naturally within us, most familiarly as dental plaque. In addition, biofilms are increasingly blamed for recurrent and chronic infections. The case is clear for the Pseudomonas aeruginosa lung infections that haunt cystic fibrosis patients and for recurrent middle ear infections of Haemophilus influenzae. But biofilms are also prime suspects in a long list of other “itises,” including endocarditis, prostatitis, and conjunctivitis.
Researchers have learned much in recent years about the mechanisms that let bacteria establish a beachhead on a surface and work together to form a highly structured matrix that nourishes and protects them. The films are easily seen by electron microscopy on foreign surfaces like catheters. However, definitively establishing a role for biofilms in a particular disease—or in chronic wounds like those of Davies’ great-aunt—is difficult, in part because traditional culture and assay techniques work best for planktonic forms. Even as the evidence comes in, however, many researchers are actively seeking unique vulnerabilities of the biofilm state, hoping to control these stubborn films wherever they occur.
A Stubborn Foe
The usual defenses often fail against bacteria that have formed biofilms. The slimy matrix protects bacteria from assaults such as those by immune cells. In addition, bacteria in a biofilm are 10–1,000 times more resistant to antibiotics than in their planktonic form.
“There’s no simple explanation why biofilms are more resistant to antibiotics,” says George O’Toole of Dartmouth. He suggests that resistance is a natural part of adaptation to life on a surface. Although people like to think that we invented antibiotics to disrupt microbial processes, most were adapted from naturally occurring chemicals that the bacteria have evolved to resist. “Almost every antibiotic that’s out there is derived from another microbe,” O’Toole says. “Bacteria and fungi have been dealing with this biological warfare for millions or billions of years.”
At first, researchers attributed the resistance mainly to the complex slime that the bacteria secrete. The slime does present a barrier to immune cells like phagocytes. However, experiments show that many antibiotics, as well as nutrients and waste products, readily diffuse through the water-rich matrix.
The slow metabolism of biofilm cells also contributes to their resistance. The bacteria in the film are relatively quiescent and divide only rarely. Antibiotics such as the penicillins, which need to be incorporated in the cell wall, are only effective against actively dividing cells. However, other antibiotics work just as well against quiescent cells, because they target basic cellular processes such as metabolism or protein or DNA synthesis. For reasons that are still being clarified, even these antibiotics are less effective against biofilms.
O, Pioneers!
Because biofilms, once formed, are so hard to eradicate, many researchers—and makers of medical devices—have tried to stop them from forming in the first place. A biofilm starts when a few pioneer cells use specialized chemical hooks to adhere to a surface. These pioneers help to make a target surface more attractive to subsequent cells, which eventually mature into a complex, structured film (Figure 1).
Makers of medical devices, such as catheters, frequently try to prepare the device surfaces to disrupt the initial adhesion. For example, they may alter the physical characteristics or chemical properties of the surface, such as its hydrophobicity, to make it harder for these first pioneers to stick. “Overall, these approaches haven’t been very successful,” asserts Phil Stewart. Stewart heads the Center for Biofilm Research at Montana State University in Bozeman. One reason for this lack of success is that surfaces immersed in bodily fluids develop a coating of bio-friendly material. Once the original surface is even partially covered, bacteria have a place to stick.
Other Approaches
Some researchers are going beyond simple surface treatments to incorporate biologically active agents into the surfaces of medical devices. Some naturally occurring proteins, like lactoferrin, interfere with bacterial adhesion. In addition, surfaces impregnated with antibiotics can delay biofilm growth, although this technique has the important downside of encouraging resistant bacteria. These surface treatments are directly useful only for human-made devices, but they also provide a testing ground for possible treatments for medical biofilms.
Strength in Diversity
Researchers are also exploiting the unique biochemistry of bacteria in biofilms, such as a lack of iron or oxygen. Once a biofilm is established, however, it develops a complex structure in which different cells occupy distinct environments. This diversity, both physiological and genetic, is an important part of biofilms’ stubbornness, say Pradeep Singh of the University of Washington. First, “the cells are experiencing different environmental conditions, so their physiology is by nature different. The guys on the outside have very different physiology from the guys on the inside, so if you have a treatment or a target or a drug that affects one, the other may not be affected.” Levels of acidity, oxygen, and iron, for example, vary widely through the film.
As the cells grow in these varying environments, they diverge genetically as well. In one study of biofilm growth, Singh says, “if we started with genetically identical population of cells, after five or ten days we’d find that population had actually diversified and was more like an old-growth forest than an monoculture of cells.” Like the forest, Singh suggests, the diversity of the biofilm could be a key element of its robust response to antibiotics and other assaults.
The diversity clearly lets biofilms recover rapidly. Researchers speak of a small population of cells, called “persisters,” that for one reason or another survive an immune or antibiotic attack. Afterwards, says Bill Costerton of the University of Southern California, “if you are a persister, you wake up in a puree of the guts of your neighbors that contains every molecule you ever needed.” These well-fed survivors can rapidly reestablish the film once the assault is over, he says. “Biofilms have a regrowth rate that is truly phenomenal.”
Dealing effectively with this diversity may require a shift from the traditional single-antibiotic approach to bacteria. “If you look at a lot of other treatment regimens for other diseases—cancer, HIV—it’s almost always a cocktail of drugs targeting different aspects of the disease process,” says Dartmouth’s O’Toole. Combining antibiotics with other compounds that disrupt the formation or survival of the film might render the bacteria more susceptible to antibiotics— and could reduce antibiotic resistance, as well.
Majority Vote
As bacteria arrange into a biofilm, the expression of scores of genes increases or decreases compared to their level in the planktonic form, raising hopes for targeting the biofilmrelated pathways. Although many of the changes are still not understood, those involved in interbacterial signaling are especially promising. Indeed, for several species, researchers have identified signaling chemicals that perform in “quorum sensing,” which induces an abrupt change in phenotype when the density of nearby cells (and thus the signaling concentration) exceed a threshold level.
At first, quorum sensing seemed to be a critical pathway for forming biofilms, although the precise pathways differ somewhat between species. Recent results, however, show that mutant bacteria that can’t do quorum sensing can sometimes form biofilms nonetheless. “The whole quorum sensing thing is still kind of shaking out,” says Montana State’s Stewart. Although he suspects that disrupting quorum sensing will remain an important tool, Stewart says, “my read at this point is it’s not as simple as ‘they have to be able to communicate to build a biofilm.’”
In addition, Washington’s Singh says that the bacteria from the lungs of cystic fibrosis patients who have longstanding biofilm infections are often mutants that can no longer perform quorum sensing. He suspects that once a biofilm is established, the chemical signals could serve as a beacon for the immune system. In their ongoing battle to evade detection, the bacteria may evolve to suppress quorum sensing when it is not helpful.
Not Just a Pretty Phage
Once biofilms develop, their most obvious distinguishing feature is the slime they secrete, which both holds the cells together and helps protect them (Figure 2). This richly structured matrix consist of a goulash of polysaccharides, as well as lipids, proteins, and even nucleic acids. Chemicals that attack this slime chemically can disrupt a variety of biofilms on medical equipment. Such a general assault is tricky for internal infections, but can be effective when applied directly to wounds.
Another strategy exploits viruses called phages that target bacteria. Phages tend to be highly specific, targeting particular species and even strains of bacteria. This specificity can be useful, especially for systemic treatments, because it can avoid disrupting natural, beneficial populations of bacteria, such as those in the gut. These beneficial bacteria often occur in biofilms themselves, and can naturally suppress more troublesome strains.
However, doctors might need to know which specific organisms causing an infection in order to choose a phage to treat it. This may not be as difficult as it seems, since a few organisms (such as P. aeruginosa, Escherichia coli, Staphylococcus aureus, and S. epidermidis) are responsible for a large fraction of infections. Researchers in the former Soviet Union have used phage to treat infections for decades and have developed cocktails that attack a spectrum of organisms. In this country, there are both practical and regulatory challenges to introducing active viruses into patients, but that may be changing.
“My sense is that increasingly the US community is open to the potential of using phage,” says Jim Collins of Boston University. With colleague Timothy Lu, Collins has used synthetic biology to alter natural viruses, adding genes for enzymes that attack the slime. The phage first hijack the bacterial machinery to replicate themselves, then break the cells open to release not just the copies, but the enzyme. Compared to the phage alone, this engineered phage “was about 100 times more effective” at disrupting a laboratory biofilm, Collins says.
Rodney Donlon heads a team at the US Centers for Disease Control and Prevention aiming to reduce infections in medical devices. He says that the engineered phage is “very interesting,” although the genetic engineering of the virus could raise further concerns about its release into the environment. Still, Donlon emphasizes that phage naturally only attack bacteria. “They will not infect human or plant cells.” But the phage are not without problems, such as the release of toxins when the cells split open or immune responses to the viruses like those that have dogged gene therapy trials.
Donlon has explored phage for devices, incorporating them into coatings on catheters. In what Donlon describes as a “proof of principle,” these coatings suppress the growth of biofilms in laboratory tests. Interestingly, they appear to remain active even after exposure to bodily fluids.
Harnessing Dispersion
Since each biofilm is different, however, researchers may need to tailor their approach to a particular species and matrix—or a combination. To overcome this problem, Binghamton’s Davies looked at the final stage of the life cycle of biofilms: autodispersal. In this stage, regions of the film spontaneously disperse as cells dissolve the matrix by secreting enzymes and revert to their planktonic form.
Davies’ team found a signaling molecule that initiates autodispersal. As in quorum sensing, this signal induces a profoundly different behavior above a threshold concentration. The chemical signals are completely different, however, and instead of causing films to coalesce, it causes them to break up.
One of the most tantalizing aspects of this molecule is that it appears to be universal across bacterial species. Although the enzymes required to degrade the matrix differ between species, the same signaling molecule triggers the process. Davies’ team has identified the molecule and verified that a synthesized version induces dispersal even in films that are below the threshold density, and works even in multispecies biofilms.
For widespread infections like those in cystic fibrosis, instantaneously releasing billions of bacteria in their planktonic form could cause even worse problems than the film. But for localized infections, like those in the sinus or middle ear, Davies thinks his dispersion-inducer could make intractable infections vulnerable to traditional antibiotics, or even to normal immune response. “Most infections are very localized, and it’s not easy for the cells to really get very far from the site of the biofilm infection,” he says. At a more personal level, Davies hopes that the dispersal agent could help millions of people with nonhealing wounds, like those of his aunt.
Dealing with Chronic Wounds
In fact, recent results show biofilms in many chronic wounds, says Randall Wolcott, who heads the Southwest Regional Wound Care Center in Lubbock, Texas. “Six months from now, I think the wound-care community will fully accept biofilm as a major barrier to healing.”
Although worried about being branded as “one of those alternative-medicine types,” Wolcott has been exploring antibiofilm therapies. “I watched so many people die, in their forties and fifties, a piece at a time,” he says. “I’d just had enough.” Overall, he says, “biofilm disease kills more people than cancer.”
For wounds that don’t respond to standard-of-care wound treatment, including mechanical removal of damaged tissue, Wolcott adds as many as six or seven agents to kill bacteria and disrupt the biofilms. He also uses phages, which qualify as natural substances and has occasionally seen “wounds that have been present for years go on to heal up in weeks.”
These ongoing studies show the potential for treatments directed at biofilms, especially in combinations that counter the natural diversity of the biofilm populations. “We know how to manage biofilms,” says Wolcott. “We just need to bring it into medicine.”
Comparison of Wound Irrigation and Tangential Hydrodissection in Bacterial Clearance of Contaminated Wounds: Results of a Randomized Clinical Study
Authors: Mark S. Granick, MD; Mayer Tenenhaus, MD; Kevin R. Knox, MD; Jason P. Ulm, MD
View/Download PDF
The process of normal wound healing involves a patterned response to tissue injury involving intricate interactions among a wide variety of cell types, structural proteins, and growth factors. These interactions frame the three recognized phases of normal wound healing — inflammation, proliferation, and remodeling. Non-healing wounds result from a disruption of this pattern and lead to prolonged hospital stays, decreased productivity, and increased healthcare costs. Many factors have been implicated in failed wound healing. Rarely is a single factor responsible for dysfunctional wound healing; usually, several interrelated factors impede the wound healing process. Wound infection is the most common cause for poorly healing surgical wounds and is often complicated by disseminated infection. The precise mechanism by which bacteria inhibit the wound healing process is not completely understood. Ahrendt et al suggest that bacteria exert their primary influence by disrupting collagen metabolism, producing and secreting various toxins, enzymes, and waste material into the wound bed. In vivo and in vitro models utilizing septic rats have demonstrated a dysregulation of collagen remodeling in the presence of bacterial endotoxin. This appears to be related to a disruption in both collagen gene expression and subsequent synthesis.
Collagen remodeling is controlled by matrix metalloproteinases (MMPs), a class of proteolytic enzymes secreted by various cells in the wound bed, including macrophages and fibroblasts. A proper balance between MMPs and their associated inhibitors is necessary for normal wound healing. In vitro studies by Okamoto et al demonstrated certain bacterial proteinases alter the activation of MMPs, affecting extracellular matrix disintegration and tissue destruction in wound healing. Bacteria also inhibit proper wound healing, generating a relative hypoxia in the local wound environment by reducing the amount of oxygen available to cells responsible for immunologic function and collagen production. This oxygen deficit can result in impaired neutrophil and macrophage function as well as improper collagen deposition.
Wound cleansing, along with thorough debridement of all necrotic tissue, is essential for bacterial clearance and a prerequisite for proper wound healing. Animal and human studies have established that a bioburden of less than 105 bacteria per gram of tissue significantly increases wound healing rates of acute wounds, whether utilizing primary or delayed closure, skin graft, or flap transference techniques.
For more than half a century, it has been well understood that thorough irrigation of wounds decreases the incidence of surgical wound infection and that this decrease is directly proportional to the volume of irrigant used. A second factor that determines the efficacy of irrigation is the pressure at which the irrigant is delivered to the surface of the wound. Numerous in vivo studies have demonstrated superior efficacy of reducing bacterial load and debris with high-powered, pulsating jet lavage in traumatic and clean-contaminated surgical wounds.
Proper irrigation of a wound includes the selection of an appropriate solution as well as the selection of a system by which to deliver that solution to the wound bed. Most commonly, normal saline with or without antibiotic preparations is used to irrigate contaminated and infected wounds. Several methods for delivering the solution to the wound surface are available, including the use of light manual scrubbing with gauze, gravity flow irrigation, bulb syringes, piston syringes, whirlpool therapy, and mechanical irrigation.
Pulse lavage (PL), first studied more than three decades ago at the US Army Institute of Dental Research at the Walter Reed Medical Center, is a delivery system that produces a pulsatile stream of irrigant powered by an electric motor. Concurrent suction provided by the device continuously removes the irrigant as well as dislodged surface pathogens, foreign material, blood clots, and necrotic debris from the wound. Although originally described for use in oral wounds, a 1978 case study by Nourse et al reported the use of PL for the irrigation and debridement of sacral pressure wounds. Since the 1970s, PL has gained universal acceptance for operative use in contaminated surgical wounds anywhere on the body and is now a standard of practice in the US for wound irrigation.
Recently, a new tool was developed for operative debridement of contaminated wounds. The high-pressure parallel waterjet (HPPWJ, Versajet [Smith and Nephew Inc., Largo, Fla]) utilizes a high-pressure waterjet oriented parallel to the surface of the wound capable of tangentially excising soft tissues at variable strengths. Th2e ability of this device to excise and remove necrotic tissue is based on the Venturi effect, a special case of Bernoulliʼs principle, which states that a fluid flowing through a tube that contains a constriction must increase in velocity through the constriction in order to decrease pressure and maintain the conservation of energy. Utilizing this physical principle, the HPPWJ creates a high-velocity stream of fluid oriented parallel to the wound surface capable of cutting through soft tissues.
The use of the HPPWJ for wound debridement was first described in 2005.30-32 The removal of debris from the wound bed is accomplished in a different manner than in PL, which utilizes an external suction force. The flow of fluid through this device generates a partial vacuum, as described by Bernoulliʼs principle, which acts to remove excised tissue and loose debris. Despite the relative novelty of the HPPWJ, it is increasing in popularity in the operative debridement of contaminated wounds. The purpose of this randomized, controlled clinical study was to compare the ability of HPPWJ to pulse lavage in reducing bacterial counts in contaminated and infected open surgical and traumatic wounds.
Methods
Setting.
The study was conducted at the University of Medicine and Dentistry of New Jersey, Newark; and the University of California, San Diego, with the authorization of the Internal Review Board at both institutions. Both HPPWJ and PL are Food and Drug Administration (FDA)-approved for use in the management of contaminated wounds.
Patients.
The patient population at both institutions present a wide variety of acute, open, surgical, and traumatic wounds. No chronic wounds such as venous or diabetic foot ulcers were included in this study.
Treatment and analysis.
Each wound was randomly assigned the method of debridement via the envelope method. In each facility, patients received clear explanations of the debridement method they would receive when informed consent was obtained. Pre- and postoperative quantitative bacterial analyses were performed on contaminated wounds that required irrigation and debridement in the operating room. The microbiology laboratories at the intuitions conducted all quantitative analyses and both laboratories were blinded to the method of irrigation and debridement. Because little correlation exists between bacterial counts from surface swabs and actual bacteria in the tissue levels, only quantitative analyses performed on tissue cultures were used in this study. Tissue samples were taken centrally from the same location in the wound immediately before debridement (in patients receiving HPPWJ treatment) or irrigation (in patients receiving PL) and immediately following completion of either debridement or irrigation. In some cases, wounds irrigated with PL also required traditional surgical debridement; post-irrigation wound culture samples were taken before surgical debridement in all cases. All post-procedure wound culture results were indexed to their corresponding pre-procedure wound culture results to yield a percentage decrease in bacterial load. This was done to account for differences in recording wound culture results between institutions. The microbiology laboratory in Newark reports wound culture results in organisms per gram of tissue; the laboratory in San Diego reports results in CFU/mL. Studentʼs t test was used to determine whether the percent decrease of absolute bacterial counts in wounds differed from pre- to postdebridement between the HPPWJ and PL groups.
Four wound specimens were excluded from analysis (two from each group) due to processing errors that lead to a prolonged incubation time, yielding aberrant quantitative results.
Results
Quantitative bacterial cultures were obtained from 25 traumatic and surgical wounds in 23 patients. Irrigation with normal saline was performed with either PL (n = 11) at a pressure of 40 lb per square inch (psi) or the HPPWJ (n = 14) at a power setting of 4 to 6 (correlating to 5,025 to 7,360 psi). As mentioned above, two specimens from each group were excluded secondary to aberrant specimen handling techniques, yielding a total of 12 wounds in the HPPWJ group and nine wounds in the PL group (see Table 1).
Bacterial counts in wounds debrided by the HPPWJ and PL decreased 90.8% and 86.9%, respectively (P = 0.38 using Studentʼs t-test) (see Figure 1and Table 2). In both groups, absolute bacterial counts decreased by an average of one to two orders of magnitude following irrigation /debridement.
Discussion
In surgical and traumatic wounds, a patientʼs ability to combat wound infection is greatly dependent on the number of bacteria present in the wound. Over the past 60 years, numerous techniques for delivering irrigant to the wound surface have been developed, ranging from the simple (eg, gravity flow and bulb syringe irrigation) to the relatively complex (eg, PL and HPPWJ).
Many studies have examined the efficacy of these various therapies. Therapy effectiveness has been found to be mainly dependent on two variables: the volume of irrigant used and the force with which the irrigant is applied to the wound surface. Although the use of larger amounts of irrigation solution is known to improve wound cleansing to a certain point, the optimal volume of irrigant remains controversial.
The optimal pressure at which the irrigant should be delivered to the wound has been studied extensively. The US Department of Health and Human Services recommends irrigation pressures in the range of 4 to 15 psi. This recommendation is based on the findings of several studies that pressures <4 psi may be insufficient to dislodge surface pathogens while pressures >15 psi may embed bacteria and particulate matter into deeper tissues, potentially causing bacteremia and traumatic damage to surrounding soft tissue, decreasing ability to fight infection. Despite these recommendations, PL is routinely used for wound irrigation at pressures as high as 40 psi.
Pulse lavage is a widely used irrigation system capable of delivering large volumes of irrigant at variable pressures ranging from 6 to 100 psi. Wounds cleansed with PL in this study demonstrated a decrease in bacterial load of 86.9%. This result confirms the work of Rodeheaver et al, who found a 84.8% decrease in contamination of experimental wounds using PL set at a pressure of 15 psi.
The HPPWJ, which has recently received FDA clearance to be marketed for wound debridement, uses an ultra-high-pressure generator to produce a high-velocity irrigant stream of variable intensity (up to 12,000 psi). The stream of saline is oriented tangentially to the wound. Any tissue affected by the stream implodes and is removed from the field by the Venturi effect. Because of the orientation of the waterjet, the HPPWJ does not embed bacteria and particulate matter into deeper tissues. Consequently, and unlike irrigation methods, the HPPWJ also removes unhealthy tissue from the wound bed. Additionally, the small nozzle and facile control offered by the HPPWJ protect collateral tissues during debridement.
Although the HPPWJ has proven to be a useful tool in the debridement of contaminated wounds, it is not without limitations. Surgeons and operating room staff need to use it several times to gain a level of comfort; inexperience can cause delays in the operating room. Excessive or inappropriate use of the device can lead to unnecessary tissue excision and exposure of underlying structures.32
In this study, use of the HPPWJ yielded a 90.8% decrease in bacterial load following the debridement of contaminated wounds. To the authorsʼ knowledge, this is the first report to quantitatively analyze bacterial clearance using the HPPWJ; thus, no comparison to previous findings is possible.
Ultimately, no difference between HPPWJ treatment and PL was found with regard to bacterial count reduction in contaminated wounds. Both the HPPWJ and PL were found to be effective in reducing bacterial load, decreasing the quantity of bacteria found in wound tissue by 90.8% and 86.9%, respectively. It should be noted that the limited size of the study restricts its power and the possibility of a type II error cannot be excluded.
The results of this study suggest that, in addition to the potential benefit of removing necrotic tissue from the wound bed, HPPWJ treatment is equal to PL in its ability to remove bacteria from contaminated traumatic and surgical wounds, which lends support to its legitimacy for use in wound irrigation.
Conclusion
High-pressure parallel waterjet and pulse lavage are equally effective in decreasing quantitative bacterial counts in infected and contaminated traumatic and surgical wounds. Studies comparing the outcomes and cost of different wound cleansing techniques using larger sample sizes are warranted.
Pressurised irrigation versus swabbing method in cleansing wounds healed by secondary intention: A randomised controlled trial with cost-effectiveness analysis
Authors: Suzanne So-Shan Mak, Man-Ying Lee, Jeanny Sui-Sum Cheung, Kai-Chow Choi, Tak-Ki Chung, Tze-Wing Wong, Kit-Yee Lam, Diana Tze-fan Lee
View/Download PDF
Abstract
Background: Wound cleansing should create an optimal healing environment by removing excess debris, exudates, foreign and necrotic material which are commonly present in the wounds that heal by secondary intention. At present, there is no research evidence for whether pressurised irrigation has better wound healing outcomes compared with conventional swabbing practice in cleansing wound.
Objectives: This study investigated the differences between pressurised irrigation and swabbing method in cleansing wounds that healed by secondary intention in relation to wound healing outcomes and cost-effectiveness.
Design: Multicentre, prospective, randomised controlled trial. Setting: The study took place in four General Outpatient Clinics in Hong Kong.
Methods: Two hundred and fifty six patients with wounds healing by secondary intention were randomly assigned by having a staff independent of the study opening a serially numbered, opaque and sealed envelope to either pressurised irrigation (n = 122) or swabbing (n = 134). Staff undertaking study-related assessments was blinded to treatment assignment. Patients’ wounds were followed up for 6 weeks or earlier if wounds had healed to determine wound healing, infection, symptoms, satisfaction, and cost effectiveness. The primary outcome was time-to-wound healing. Patients were analysed according to their treatment allocation. This trial is registered with ClinicalTrials.gov, number NCT01885273.
Results: Intention-to-treat analysis showed that pressurised irrigation group was associated with a shorter median time-to-wound healing than swabbing group [9.0 days (95% CI: 7.4–13.8) vs. 12.0 (95% CI: 10.2–13.8); p = 0.007]. Pressurised irrigation group has significantly more patients experiencing lower grade of pain during wound cleansing (93.4% vs. 84.2%; p = 0.02), and significantly higher median satisfaction with either comfort or cleansing method (MD 1 [95% CI: 5–6]; p = 0.002; MD 1 [95% CI: 5–6]; p < 0.001) than did swabbing group. Wound infection was reported in 4 (3.3%) patients in pressurised irrigation group and in 7 (5.2%) patients in swabbing group (p = 0.44). Cost-effectiveness analysis indicated that pressurised irrigation in comparison with swabbing saved per patient HK$ 110 (95% CI: 33 to 308) and was a cost-effective cleansing method at no extra direct medical cost with a probability of 90%.
Conclusions: This is the first randomised controlled trial to compare the pressurised irrigation and swabbing. Pressurised irrigation is more cost-effective than swabbing in shortening time that wound heals by secondary intention with better patient tolerance. Use of pressurised irrigation for wound cleansing is supported by this trial.
What is already known about the topic?
- Wound cleansing is an important part of assisting the wound to heal by secondary intention; by removing foreign debris and excess exudate, reducing bacterial bioburden and rehydrating the wound
- Swabbing is a dominant practice in wound cleansing despite the mention about its risk for tissue trauma thus compromising healing.
- Pressurised irrigation has been advocated as an acceptable practice to cleanse wounds, due to its merit in being able to cleanse without traumatising the wound bed.
What this paper adds
- Pressurised irrigation has better wound healing outcomes including shorter wound healing time, less pain during wound cleansing, and higher satisfaction with comfort and the cleansing method compared with swabbing practice to cleanse wound.
- Pressurised irrigation is a cost-effective alternative to swabbing for cleansing wounds that heal by secondary intention.
- This study is the first with randomised controlled trial design to compare the irrigation and swabbing, while accounted for cost analysis, which previous studies had not done.
1. Introduction
A wound heals by secondary intention if surgical closure is not indicated by reason of wound edges being unable to approximate due to tissue loss and wound being contaminated or infected, including acute traumatic wounds (Dire and Walsh, 1990), dehisced surgical wounds (Miller and Glover, 1999), chronic wounds (Falanga, 2000), leg ulcer (Waspe, 1996) and burn wound (McKirdy, 2001). By secondary healing, the wound is allowed to ‘‘granulate in’’, that is, the wound closes by contraction and filling with connective tissue, which may be a protracted process, more nursing time in managing the wound will be required. Wound cleansing is an important part of assisting this healing process; by removing foreign debris and excess exudate, reducing bacterial bioburden and rehydrating the wound (Atiyeh et al., 2009; Falanga, 2000).
The most appropriate technique of wound cleansing remains contentious over the years. The routines for cleansing wounds vary between countries, hospitals and departments, some literatures recommend not to use swabbing routinely due to the risk for tissue trauma thus compromising healing (Oliver, 1997), while others recommend swabbing with soaked non-woven gauze at appropriate pressure which can remove slough and loose necrotic tissue without damage (Towler, 2001; Young, 1995). In Hong Kong, the use of swabbing in cleansing wounds is a dominant practice in majority of healthcare setting despite the availability of literature and expert recommendation.
A number of narrative review articles have indicated various techniques for wound cleansing. However, irrigation of wounds is gaining widespread acceptance as clinicians recognise its benefits, namely preservation of newly granulating tissue, effective removal of bacteria and debris and patient comfort and convenience (Ennis et al., 2004; Oliver, 1997). The original Agency for Health Care Policy and Research (AHCPR) guidelines describe safe and effective irrigation pressures as being 4–15 psi, based on a series of different studies (Brown et al., 1978; Rodeheaver et al., 1975; Wheeler et al., 1976). Studies suggest that pressures of 8–12 psi are strong enough to overcome adhesive forces of bacteria (Chisholm et al., 1992; Longmire et al., 1987). Use of pressurised irrigation facilitates ease of irrigation, markedly decreases the time involved in this traditionally labour-intensive activity, and may decrease budgetary burden due to extra add needles or syringes for irrigation.
Since cleansing by irrigation being considered advantageous, there has been a lot of debate and research with regards to the most appropriate equipment and amount of pressure required to effectively cleanse a wound without causing trauma (Towler, 2001). No study that compared the technique of swabbing with either irrigation or pressurised irrigation was identified from the updated search.
A Cochrane Wound Group’s review concluded that there were no randomised controlled trials identified that compared the common techniques of swabbing and scrubbing (Fernandez et al., 2006; Moore and Cowman, 2013). The conclusions in the Cochrane review were based on the Joanna Briggs Institute Best Practice report that the data were analysed using Cochrane Review manager, showing that there were only five trials comparing the effect of showering to non-showering patients in the post-operative period (Fernandez et al., 2006). The pooled results of four studies (Fraser et al., 1976; Goldberg et al., 1981; Riederer and Inderbitzi, 1997; Voorhees and Rosenthal, 1982) indicated that there was no statistical difference in the infection rate (OR = 0.80; 95% CI = 0.29–2.21) and the healing rate between the groups. However, two studies reported that patients who were in the showering group felt a sense of health and well-being derived from the hygiene and motivation of showering (Riederer and Inderbitzi, 1997; Voorhees and Rosenthal, 1982). A Cochrane review for wound cleansing of pressure ulcers identified only a small randomised controlled trial showing a statistically significant reduction in volume reduction in pressure ulcers cleansed with pulsatile lavage (MD 6.60, 95% CI: 11.23 to 1.97) compared with those cleansed using sham pulsatile lavage, and thus emphasised that well designed, robust studies are required (Moore and Cowman, 2013). By evaluating both healing outcomes and cost-effectiveness, a more complete overview of the wound cleansing by pressurised irrigation and swabbing can be obtained and used as guidelines. These guidelines can serve as a common repository of generally accepted practice.
2. Methods
2.1. Study design and participants
This was a multicentre, randomised controlled trial that took place in four General Out-patient Clinics (GOPC) in Hong Kong. Participants were identified from the GOPC at their visit for dressing treatment. Eligible patients were those with wounds in any type to heal by secondary intention, speaking Chinese, with an abbreviated mental test score 7 or above indicating their normal cognitive ability; and being able to be accessible for wound cleansing and evaluation follow up. Exclusion criteria included unbroken skin; full-thickness skin loss and damage to muscle, bone or/and any supporting structures; wounds with a sinus; wounds to heal by primary intention including adhesive strips, sutures or super glue; wound that was prescribed to be cleansed by irrigation; and patients with a very poor life expectancy or with a clinical condition that severely interfere with wound healing such as malignancy, autoimmune disease. All patients provided written informed consent for trial participation.
2.2. Randomisation and masking
We enrolled patients and randomly allocated them to either pressurised irrigation or swabbing before wound cleansing. The group allocation of each participant was assigned by having a staff independent of the study opening a serially numbered, opaque and sealed envelope to ensure concealment. The envelopes containing the group identifier were prepared by a statistician blinded to the study using computer generated random codes prior to subject recruitment.
Patients and operators were aware of treatment allocation, the trial staff performing data collection and wound assessment was masked to treatment group.
2.3. Intervention
For patients allocated to the study group, wounds were cleansed with pressurised irrigation technique using a pressurised irrigation device (Fig. 1) which was modified by connecting an instrument DeVilbiss Syringe, to Gomco’s1 Vacuum/Pressure Pump Model 309, generating a steady irrigation stream at a consistent range of impact pressure from 4 to 13 psi. The Syringe is a small flexible tube with opening in forward end furnished with a bottle to hold liquid, which permits deep yet painless lavage.
Pressurised irrigation group received the ‘standardised usual care’ the same as those in the control group that had wounds cleansed with swabbing technique using forceps and cotton wool (in sterile dressing pack). The ‘standardised usual care’ included cleansing wound with normal saline solution at room temperature; used saline to be dated and used within 24 h after opening; selecting wound dressing according to the protocol of wound management in GOPC; all dressings being kept intact until next visit; and amount of saline used and frequency of dressing change depending on the amount of exudates.
The wound care practice in GOPCs was guided by the protocol consisting of three basic elements in wound management: cleansing techniques, cleansing solutions and dressings. Since the cleansing techniques were the key aspect this study was testing, only the standard care about cleansing solution and dressing used were addressed. Normal saline is isotonic that is the most commonly and safely used to cleanse wound. The principle of dressings selection is guided bymoistwound theory inkeepingwound moist and controlling exudate, as well as availability of the dressings that a variety types of dressing materials, e.g. alginates, hydrofiber, hydrocolloid, foam are usually available in the GOPCs.
All wounds were cleansed following the allocated method until the wounds were completely healed or for 6 weeks if the wounds had not yet healed.
2.4. Data collection
Data collection and wound assessment took place for all subjects at enrollment and upon healing of the wound or at the end of 6-week period if the wounds had not yet healed. Wounds that had not healed at the end of the 6- week period were reassessed and data relating to the wound characteristics were recorded. The operators who undertook dressing change were responsible for the ongoing assessment of the wound during cleansing and recording the information on the volume of solution and amount of cleansing materials used, frequency of dressing changes and the type of dressing applied at each visit.
Data for checking baseline differences and data related to wound healing problems were abstracted from the medical records. The collected data included age, sex, body weight & height, history of smoking, medical history, concomitant medication, current treatment and abbreviated mental test (AMT). Bates-Jensen Wound Assessment Tool (BWAT) (Bates-Jensen, 2000, 2001) comprising 13 assessment scored items (size, depth, edges, undermining or pockets, necrotic tissue type, necrotic tissue amount, exudate type, exudate amount, surrounding skin colour, peripheral tissue oedema, peripheral tissue induration, granulation tissue, and epithelialisation) was adopted to capture the baseline wound features, and the lower scores indicates a healthier wound.
2.5. Clinical outcome measures
The primary outcome was time-to-wound healing defined as number of days from recruitment to complete healing which was indicated by complete coverage of the wound with epithelial tissue. Patient’s wound that was observed to completely heal was verified by trial nurse who was masked to treatment allocation.
Secondary clinical outcomes included proportion of wounds completely healed and reduction of wound size during the 6 weeks oftrial participation; presence of signs of wound infection and physician prescription of antibiotic for a wound infection at any time up to 6 weeks after randomisation. Visitrak digital planimetry system (Thawer et al., 2002) was used to measure and document the dimensions and attributes of wound, to ensure an objective, accurate and reproducible evaluation of wound size. Operator identification of signs of wound infection was verified by contacting a physician who was masked to treatment allocation to confirm prescription of an antibiotic for wound infection.
2.6. Other effectiveness outcome measures
Patient’s symptoms and problems related to the wound were measured at enrolment and upon healing of wound or at the end of 6-week period if the wounds had not yet healed by using the self-rating scale of Wound Symptoms Self-Assessment Chart (WoSSAC) (Naylor, 2002). This WoSSAC divide wound symptoms into six aspects (pain from wound, pain related to dressing changes, fluid leakage from dressing, bleeding, smell, itching). Each of these aspects has two dimensions (severity and impact on patient’s life) to be assessed.
Patient satisfaction related to wound cleansing and health-related quality of life were measured at the end of the patients’ participation in the study. We used two self-rating questionnaires: a self-devised satisfaction survey which had 6-point scale with anchoring description at each of the points to measure patient’s satisfaction with the cleanliness, comfort with wound cleansing, and overall satisfaction; and a generic health-related quality of life measure, SF12 (Lam, 2001; Lam et al., 2005).
A list of cost measurements for the wound cleansing was captured. The duration of the wound dressing performed in each visit, amount of follow-up and amount of dressings, solution and equipment used in cleansing were documented. Patient had a card on which the amount of solution and the amount of dressings required for cleansing were recorded when they attended the appointment for wound assessment in other hospitals.
2.7. Sample size
Previous studies have shown 40% (p1) normal saline irrigation-cleansed wounds in community health centres healed completely within the 6-week period. In order to have 90% power, with a two-side 5% level test, to detect a 20% (d) p2 improvement in the healing of wound in the irrigation device arm as compared to the control practice arm (i.e., an increase to 60% (p2) wounds healed within 6 week), we needed about 97 patients in each arm. The formula was as follow: (see pdf).
Considering about 25% of patients loss to follow-up or withdrawn from this trial, the sample size was inflated to 122 patients in each arm.
2.8. Statistical analysis
All the main primary and secondary outcome measures, except patient satisfaction and health-related quality of life, were analysed on the basis of intention-to-treat (ITT) principle. In view of the gradually improving nature of these outcome measures, missing outcome data, except time-to-wound healing, were imputed using last observation carried forward approach, where more conservative efficacy results would generally be obtained. In the survival analysis of time-to-wound healing, the dropped-out cases owing to adverse events were considered as having unfavourable outcome (incomplete wound healing) throughout the study period (6 weeks) if they have not reached the endpoint before the occurrence of adverse events. All other dropped-out (lost to follow-up) patients were considered as censored cases in the survival analysis.
Time-to-wound healing was estimated by Kaplan– Meier method and compared between pressurised irrigation and swabbing using log rank test. Furthermore, Cox proportional hazards model was used to estimate the hazard ratios of the irrigation group versus swabbing group on time to wound healing with and without adjustment for covariates, including initial wound size, receiving antimicrobial treatment at the baseline and leg ulcer wound. These covariates are supposed to affect the progress of wound healing. Proportion of wounds completely healed and infection rate during follow-up as well as patient perceived wound symptoms at study completion were compared between the two arms using Pearson chi-square test or Fisher’s exact test, as appropriate. Reduction of wound area and percentage of reduction were both assessed using Mann–Whitney test. Patient satisfaction and health-related quality of life scores were compared between the two arms in the per-protocol population using Mann–Whitney test and independent t-test respectively.
Since it is difficult to make justifiable imputations to the dropped-out cases, particularly, for cost data, costeffectiveness analysis of wound healing with pressurised irrigation in comparison with swabbing was therefore performed in per-protocol population only. Total direct medical cost of wound dressing per patient was estimated for each treatment method by arithmetic mean (Thompson and Barber, 2000). Mean time-to-wound healing estimated by the approach of Efron (Efron, 1967) was adopted as the effectiveness measure. Biased-corrected and accelerated bootstrapping with 5000 replications (Efron, 1987) was used to estimate confidence intervals of mean difference in the medical cost and time-to-wound healing between the two arms. Mean cost difference between the two arms (pressurised irrigation – swabbing) and mean difference in time-to-wound healing between arms (swabbing – pressurised irrigation) were calculated to represent, respectively, the incremental cost and incremental effect of the pressurised irrigation over swabbing. The bootstrapped 5000 pairs of incremental cost and effect data were plotted on a cost-effectiveness plane to graphically illustrate their uncertainties. Cost-effectiveness acceptance curve (Fenwick et al., 2004) was generated to demonstrate the probability of cost-effectiveness of pressurised irrigation over swabbing at different thresholds for willingness-topay for saving one day to complete wound healing.
The bootstrapping was performed using Matlab 7.0 (The Mathworks, Inc). All other statistical analyses were done using SPSS 20.0 (SPSS Inc., Chicago, IL). All statistical tests were two-sided and a p value <0.05 was considered statistically significant.
3. Results
Between April 2008 and August 2010, we screened 502 patients and randomly assigned 256 patients to have wound cleansed by the pressurised irrigation (122 patients) or the swabbing (134 patients). 45 eligible patients were not enrolled due to twice suspension of study, caused by emergence of the human swine influenza case in HK between May 2009 and July 2009; and implementation of vaccine programme in GOPCs between October 2009 and February 2010. 39 (15.2%) of 256 patients included in the analysis could not complete this trial, because 30 patients (15 patients in pressurised irrigation and 15 patients in swabbing) were lost to followup mainly owing to majority being male who defaulted visit and rushed to back to work before wounds healed; and adverse events occurred in 9 patients (Fig. 1).
The wounds that have been recruited were all by secondary healing, including trauma wound, i.e. laceration/abrasion, burns/scalds, dehisced surgical wound, leg ulcer, dog bite, etc. Trauma wound accounted for nearly one third (30.1%) of the wounds, followed by burns/scalds (17.6%) and dehisced surgical wounds (17.2%). The most common anatomical regions of wound were lower extremity, followed by upper extremity, trunk and head or neck.
Baseline demographic and clinical characteristics in the two study groups were well balanced except for a slightly more female in the pressurised irrigation group (Table 1). A higher proportion of patients in swabbing group (5.9% vs. 0.8% in pressurised irrigation group) developed adverse events thus requiring change of wound treatment that all of them were not deemed directly related to the used cleansing technique (Table 2).
3.1. Primary outcomes
Kaplan–Meier estimates of median time-to-wound healing was 9.0 days (95% CI: 7.4–10.6 days) in the pressurised irrigation group and 12.0 days (95% CI: 10.2– 13.8 days) in the swabbing group (p = 0.007, log rank test) (Fig. 2 and Table 3a). Based on Cox proportional hazards model, the unadjusted hazard ratio (HR) of irrigation group versus swabbing group on time-to-wound healing was 1.44 [95% confidence interval (CI): 1.09–1.89; p = 0.010]. Using hierarchical Cox regression modelling, the adjusted HRs (95% CIs) of irrigation group versus swabbing group on time-to-wound healing were, respectively, 1.43 (1.09–1.89), p = 0.011; 1.35 (1.02–1.79), p = 0.034; 1.29 (0.97–1.70), p = 0.077, with adjustment for successively adding covariates (1) initial wound size, (2) receiving antimicrobial treatment (yes/no) and (3) leg ulcer wound (yes/no) into the model (Table 3b). Regarding the other primary outcome, there was no significant difference found in proportion of wounds completely healed after 6 weeks between the two groups (Table 3a).
3.2. Secondary outcomes
The proportion of wounds to heal completely before the end of the 6-week study period was 82% in the pressurised irrigation group and 78.4% in the swabbing group, however difference was not statistically significant. Majority of wounds decreased in size over the study period. The reduction in wound size did not differ significantly between groups (Table 3a). An increase in the size of a wound that dermatitis happened on the skin around the wound was noted in the control group, which was then improved after steroidal treatment started. The overall wound infection rate during follow up was 3.7%. Incidence of wound infection up to 6 weeks after randomisation did not differ significantly between groups (Table 3a).
Lower grade of pain experienced during wound cleansing was more frequent in the pressurised irrigation group than in the swabbing group (93.4% vs. 84.2%; p = 0.02), but the level it interfere less with patients’ life was similar (95.1% vs. 91.0%; p = 0.201). Other adverse symptoms (pain on wound; fluid leaking from wound cleansing; wound bleeding; wound smell; itchiness on wound or surrounding skin) at any grade and the level they interfere with patients’ life correspondingly were not different between groups (Table 4).
Patients allocated to pressurised irrigation had significantly higher satisfaction with comfort after wound cleansing and wound cleansing method than did patients allocated to swabbing, but the satisfaction with cleanliness after wound cleansing did not differ between groups (Table 5). Patient generic health-related quality of life did not differ between groups during follow-up (Table 5).
3.3. Cost analysis
In the 6-week follow-up period, mean total direct medical cost per patient in swabbing and pressurised irrigation groups were respectively HK$ 354 (SD 882) and HK$ 244 (SD 283) in per-protocol population (Table 6). The total direct medical cost did not differ between groups based on the bootstrapped 95% confidence interval. The cost of cleansing wound with pressurised irrigation saved per patient was HK$ 110 (95% confidence interval: HK$ 33 to 308) compared to the swabbing. The mean time-towound healing in the swabbing and pressurised irrigation groups were respectively 14.5 and 11.4 days. On average, cleansing wound with pressurised irrigation could save 3.1 days (95% confidence interval: 0.3–5.9 days) to complete wound healing when compared to swabbing. The costeffectiveness plane (Fig. 3) displays the distributions of the incremental cost and effect data of the bootstrapped results with 5000 replications. The majority (90%) of the bootstrapped cost-effectiveness pairs were located in the south-east quadrant, indicating that the pressurised irrigation was dominantly more effective and less expensive than the swabbing method. The cost-effectiveness acceptability curve (Fig. 4) shows the probability of costeffectiveness of the pressurised irrigation in comparison with swabbing versus the ceiling amount of willingnessto-pay for saving one day to complete wound healing. The probabilities of cost-effectiveness of the pressurised irrigation in comparison with swabbing were 90%, 95% and 98% at willingness-to-pay an extra of HK$ 0, 8 and 28 respectively per patient per one day saved to complete wound healing.
4. Discussion
4.1. Uniqueness of the study
This paper is the first reported randomised controlled trial comparing swabbing and pressurised irrigation as techniques for cleansing wound, which has shown pressurised irrigation applied to wounds healed by secondary intention, is safe, and more cost-effective in shortening the healing time of wound. However, it is worth noting that the hazard ratio of pressurised irrigation group against swabbing group on wound healing became only borderline significant [HR = 1.29 (95% CI: 0.94–1.70), p = 0.077] after adjusting for initial wound size, receiving antimicrobial treatment or not and leg ulcer wound or not. In fact, the study sample consisted predominantly of acute wounds and a relatively higher proportion of participants in swabbing group with chronic leg ulcer than in the irrigation group (7.5% vs. 1.6%) might explain the lost in significance in the adjusted analysis. Future research and trials are recommended to replicate the study particularly in chronic wound populations. Nevertheless, patient presented less pain during wound cleansing over the course of treatment; and reported higher satisfaction with comfort after wound cleansing and with cleansing method. There was no clinically important difference in the variation of wound infection rates between two groups.
The results agree with narrative review about benefits of irrigation namely promoting wound healing and patient comfort (Oliver, 1997); and shortcoming of swabbing that extra pressure applied on to the wound has repeatedly been shown to have deleterious effects on tissue and thus the healing of wounds (Miller and Glover, 1999; Oliver, 1997). This result echoes with that of the Ho’s trial (Ho et al., 2012), which demonstrated a statistically significant reduction in volume of pressure ulcer cleansed with pulsatile lavage at 11 psi of pressure compared with those cleansed using sham pulsatile lavage. However, Ho’s trial was a small study and these between group differences did need to be confirmed in a larger study.
Presence of pathogens in a wound microbiological culture is not, in itself, indicative of clinical infection (Cutting and Harding, 1994), which would not enhance methodology or inform the results. We therefore used a primary patient-assessed wound infection signs and symptoms backed by physician action of antibiotic prescription. This analysis was designed to reflect usual clinical care and experience, with less bias to capture relevant events. 23.8% of patients were assessed to have wound infection requiring antibiotics treatment at entry of study, suggesting a higher initial infection rates in our sample due to all recruited wounds healing by secondary intention that a large number of pathogenic flora usually colonise there (Miller, 1996). Majority of the wounds had infection resolved then and overall wound infection rate fell to 3.7% during follow up. Griffiths et al. (2001) reported higher wound infection rates of 6.1% in patients followed up in community health centres; however, it compared the effects of irrigation by tap water and normal saline, and the sample size was small. Moscati et al. (2007) reported similar wound infection rates of 3.65% in patients with acute lacerations treated in emergency departments; however, it compared tap water versus sterile saline for wound irrigation.
The pressure used for irrigation has repeatedly been shown to be an important variable in the infection rates of wounds. The pressurised irrigation device (Fig. 5) modified from the already long available but decreasingly used equipment in Hospital Authority hospitals, was able to generate steady irrigation stream at pressure from 4 to 15 psi which was purposively to be tested in this study. The glass bottle and stainless steel nozzle were reusable between patients, and they were cleansed and autoclaved after use every 24 h. Although samples of saline from the reusable glass bottle and stainless steel nozzle were not subjected to laboratory analysis to determine if there was contamination, our results did not demonstrate significantly increased infection rates in cleansing wound using the self-modified irrigation device for irrigation.
Most notably, nine adverse events were reported, eight from the swabbing arm. Three of them developed wound complication such as tunnelling and abscess requiring further surgical intervention. Two patients were prescribed to change to irrigation method during hospitalisation and doctor consultation and three were prescribed with betadine solution for wound cleansing. Need for change of treatment did imply the wounds were difficult to heal. It is possible that some wound infection may have occurred in the group. Removal of them from the analysis might contribute to underestimate the infection rate in swabbing group.
4.2. Strengths and limitations
Our trial is the first with randomised controlled trial design to compare the pressurised irrigation and swabbing. It was designed to minimise confounding factors that could influence outcomes and test the wounds to be healed by secondary intention despite acute or chronic. Our results from a larger sample size and multi-centre comparison of wound cleansing technique should be more generalisable. Although recruited numbers of participants varied in the four GOPCs due to the environmental factors when the trial conducted, the proportion of recruited patients between groups was similar in each centre. The bias in outcome assessment has been minimised by having assessor who was different from the operator undertaking dressing change in this study, and instructing the trial staff who performed assessment not to ask about the method of cleansing during patient follow-up visit. The pragmatic design of our trial, sufficiently powered sample size, and use of primary outcomes combining perspectives from patients and cost expense on the wound cleansing technique, provide clear evidence for the cost-effectiveness of pressurised irrigation in shortening the healing time of wound that heals by secondary intention and superior patient tolerance compared with swabbing.
The trial has some limitations: moderate compliance in returning back for assessment (24 (80%) of 30 patients lost to follow-up were male in working age); no masking of patients; imperfect masking of the assessors (because of some patients talking their allocated treatment to assessors, which could bias detection of our primary outcome); no masking of operators (which might bias their use of background treatment); unpredictable trial suspension in a short notice affecting recruitment in a few of GOPCs; and our data contain a lower proportion of chronic type of wounds. Although the trial protocol did not intentionally select for certain wound types, recruitment of a higher proportion of acute wound (trauma wound, burns/scalds and dehisced surgical wound) was probably attributable to region demographic characteristics that there was large population performing labour work and thus more vulnerable to injury such as cuts or scald. This might bias the estimate of the effect of pressurised irrigation. Other potential limitation included imbalance in the background use of wound dressing materials but we deem this is unlikely to have introduced bias or altered the external validity of the results.
4.3. Implications and explanations of findings
We noted the basic cost of wound cleansing materials per patient was less for wounds allocated to pressurised irrigation than wounds allocated swabbing. While the average dressing materials (dressing fixation materials, supplementary dressing materials) and labour cost per patient were also lower for pressurised irrigation. All of these contribute to a lower total direct medical cost in the pressurised irrigation group. It was however a great variance in the total direct cost for swabbing was reported. This may be attributable to variations in costs especially for supplementary dressing materials among the subpopulations with different wound types. Costs are considerably higher for chronic wounds than acute wounds, which may therefore create great variability of central tendency in the costing analysis. This indicated we would need more subjects with wounds taking longer to heal, i.e. chronic wound for sufficient power to detect difference in outcomes between groups. Considering the widespread use of swabbing for wound cleansing in the community, more large high quality randomised controlled trials of the wound cleansing techniques are warranted.
Pressurised irrigation may also have advantage of potential cost saving at indirect cost over conventional swabbing technique, although the effect is difficult to analyse fully. The dressing packs used extensively in swabbing, however, generates unnecessary waste from the disposal of unused items—swab, gauze and wrappings; and items that have reusable alternatives—forceps or tray. Landfill space is so scarce in HK where the three existing landfills are full in the mid to late 2010s (GovHK, 2013). As a result, landfill disposal fees are increasing per year. These financial and environmental liabilities of waste disposal make reducing the quantity of non-hazardous waste imperative. The self-modified pressurised irrigation device tested in this trial will represent a prototype model to demonstrate how effective wound irrigation can be performed without using sterile dressing pack. The hospital pays $4.5 for each dressing pack. Specific substitutions in the dressing packs may yield a net cost savings which is albeit seemingly low. The difference is huge when applied to over 1 million wounds treated in all cross-cluster GOPCs each year as well as counting expensive fee of landfill, furthermore adding to the additional total direct medical cost in wound dressing change (HK$ 110 more in the swabbing group). There is a need to further analyse the indirect cost between the cleansing methods.
5. Conclusions
This study suggests that wound cleansed by pressurised irrigation method is more cost-effective in shortening the healing time of wound that heals by secondary intention; moreover, pressurised irrigation is highly well tolerated by patient presenting less pain during wound cleansing, and higher satisfaction with comfort and the cleansing method. Importantly, pressurised irrigation is not associated with an excess of major adverse events and wound infection, reassuring about its safety in the community population with a variety types of wounds.